Department of Chemical Engineering and Bioprocesses
Portada » Bioplastics produced by bacteria: a genetic model to make them more competitive

Department of Chemical Engineering and Bioprocesses

Department of Chemical Engineering and Bioprocesses
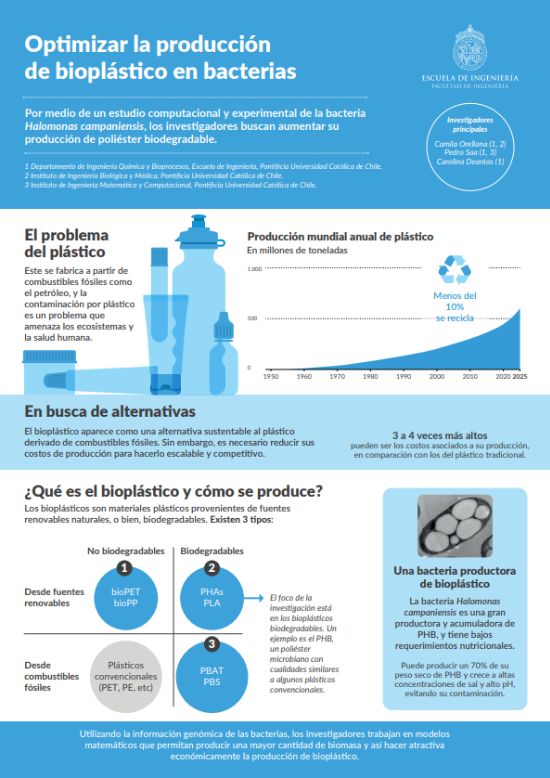
Plastic pollution is one of the most serious environmental problems globally, affecting wildlife, oceans, and human health. Despite growing awareness of its consequences, petroleum-based plastic remains an integral part of daily life, and its use continues to rise. Of all the plastic ever produced, 56% has been manufactured in the last 20 years, and currently, less than 10% is recycled. Projections indicate that plastic production will continue to grow exponentially, reaching nearly 1.5 billion tons by 2050.
In response to this challenge, the research team led by Camila Orellana has identified a potential solution in bioplastics. Specifically, their work focuses on the ability of the bacterium Halomonas campaniensis to produce microbial polyester (PHB), a material that shares properties with conventional plastics such as polyethylene (PE) and polypropylene (PP), but that naturally breaks down in the soil through the action of microorganisms, preventing its accumulation in the environment.
Image of Halomonas sp. showing intracellular PHB granules. Source: Tanaka et al. (2022), Polymers, 14(15), 3063. Licensed under CC BY 4.0.
A key challenge: Reducing costs
One of the main challenges in producing bioplastics is the high cost involved—PHB production is three to four times more expensive to produce than conventional plastics. The bacterium Halomonas campaniensis has several characteristics that make it particularly attractive for study: it requires few nutrients, can use various substrates as carbon sources, is able to grow in open cultures using saltwater without contamination, and can produce up to 70% of its dry weight in PHB. These traits help reduce the production costs of PHB, but it’s still not enough. For this reason, Camila Orellana and her team, in collaboration with Professor Pedro Saa, aim to boost PHB production in these bacteria to enhance the economic competitiveness of this bioplastic.
HaloGEM: A model to optimize production
Broadly speaking, their approach starts with decoding the bacterium’s genome—that is, identifying every gene within its DNA. This genetic information reveals which enzymes and proteins the bacterium can produce, crucial components that enable the diverse chemical reactions occurring inside the cell. Using this data, the researchers built a computational model called HaloGEM (Halomonas Genome-scale Metabolic model), which maps all the metabolic pathways the bacterium can potentially activate.
These metabolic pathways act as biochemical roadmaps, illustrating how the bacterium transforms nutrients such as glucose and salts into energy, biomass, and valuable compounds like PHB. With HaloGEM, researchers can run virtual simulations of different growth conditions or “in silico experiments” to predict which nutrient combinations or genetic tweaks would boost bacterial growth or increase bioplastic production. This method provides a strategic guide to designing real-world experiments, grounded firmly in the bacterium’s own biology.
Schematic overview of how HaloGEM operates. Using the genome of Halomonas campaniensis, this computational model reconstructs the bacterium’s metabolic pathways and simulates their activity under various conditions. This enables HaloGEM to predict the most efficient pathways for boosting production of the bioplastic PHB, guiding the design of optimized culture media and targeted genetic modifications to enhance yield.
To validate HaloGEM’s predictions, the team modified the culture medium and tested different formulations. Starting from a baseline medium, they developed and evaluated six different formularions based on the most promising metabolic pathways identified by the model. The results were striking: the optimal medium—which included glutamate and arginine as nitrogen sources, glucose as a carbon source, along with vitamins and salts—increased bacterial biomass by 54% and PHB production by 153% compared to the control. Furthermore, HaloGEM identified specific genes that, if modified, could lead to an even greater increase in production.
Department of Chemical Engineering and Bioprocesses

The results of this study represent an important step toward more efficient bioplastic production. Still, the team’s work continues. The next phase focuses on further reducing costs by using more accessible and sustainable resources, such as agro-industrial waste instead of glucose, seawater as a solvent, and targeted genetic modifications suggested by the HaloGEM model. These efforts could help make PHB a more competitive option for producing everyday items like bags, bottles, or containers that naturally degrade in the environment.
Imagining a future where plastics are not an environmental threat but part of the solution remains an achievable goal. Camila Orellana and her team offer a valuable contribution in this direction, demonstrating how scientific knowledge can open new pathways to tackle urgent challenges. Although there is still work to be done, this research invites us to rethink the materials we use and their impact on the planet. Perhaps, in this process, bacteria may play an unexpected role in the transition toward more circular and sustainable production.
Towards a Greener Future

Department of Chemical Engineering and Bioprocesses